Can You Change the Number of Neutrons in an Atom
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. An ionic bond D.
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
The variation of the number of neutrons in.

. The addition of a neutron can make an atom radioactive. An anion C. If you change the number of neutrons in an atom you create _____.
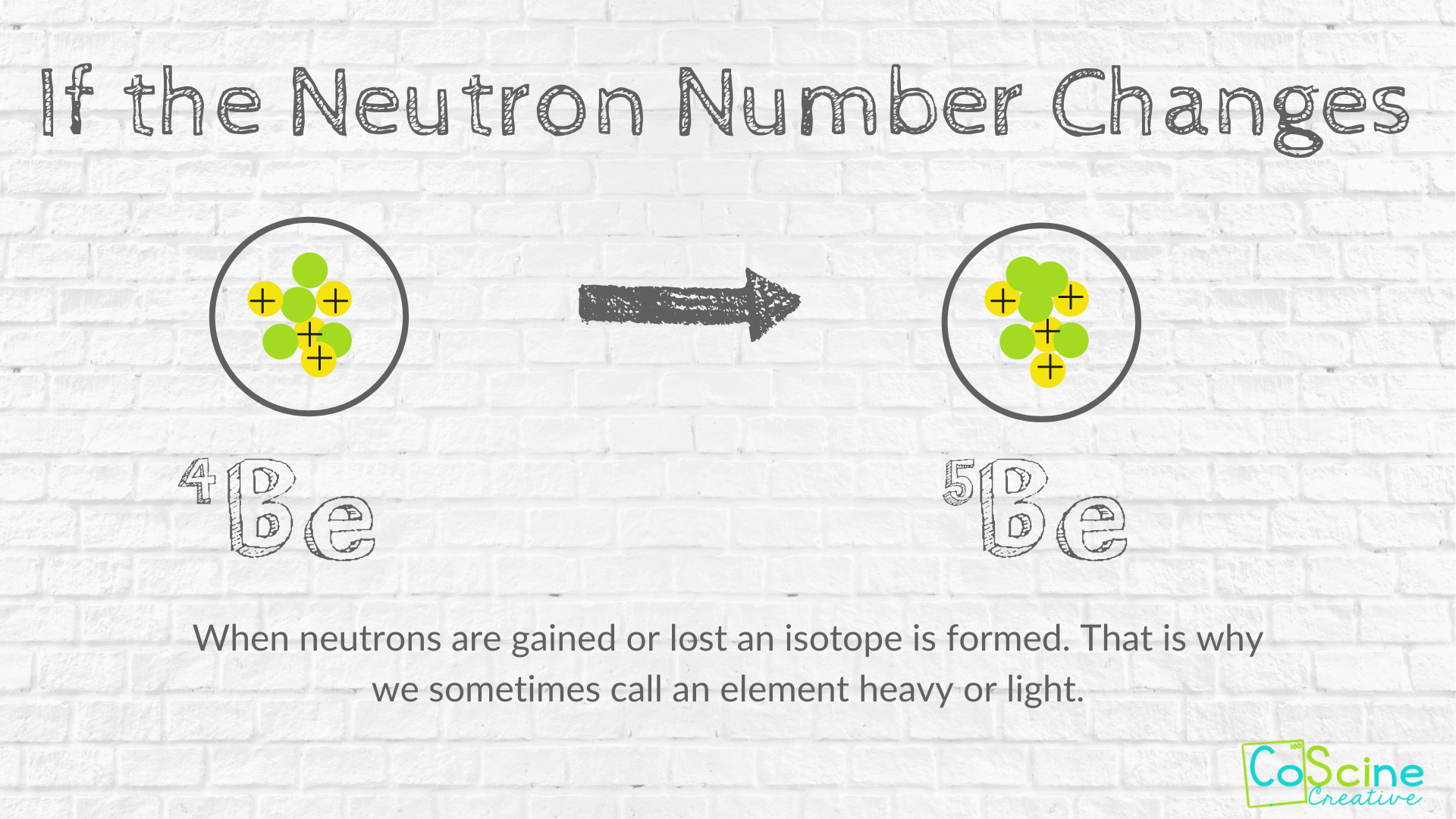
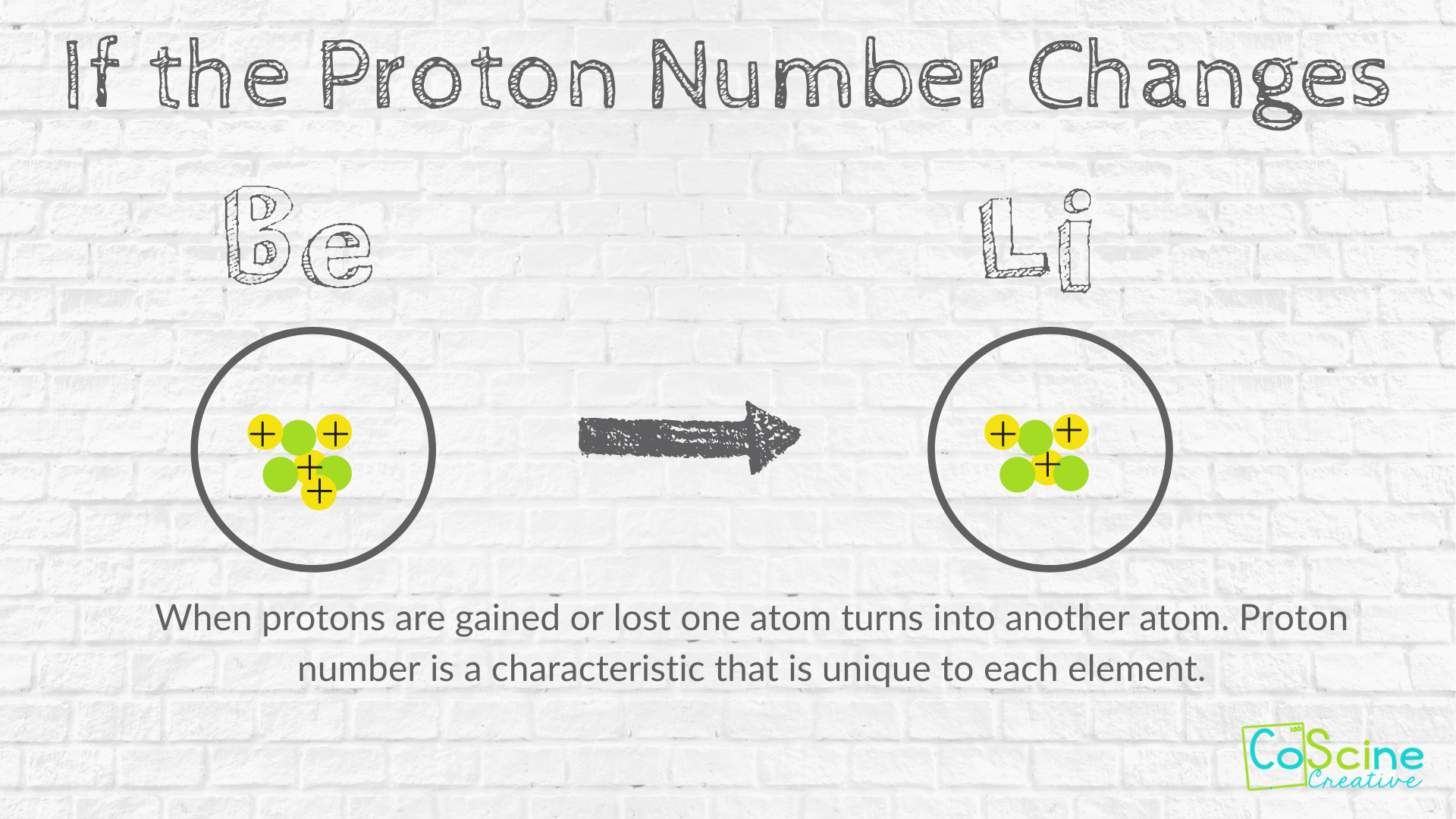
If you change the number of neutrons in an atom you get an isotope of the same element. When you change the number of protons in an atom you will change the atom from one element to a different element. Also to know is how does changing the number of protons in an atom affect.
The subatomic particle that never changes is the electron. The addition of a neutron can make an atom radioactive. Can an atom lose a neutron.
Therefore you can subtract the atomic number from the mass number to find the number of neutrons. A An atom is a solid mass of material. Protons and neutrons are tightly bound to the nucleus.
2 When you change the number of you change the element. If you change the number of neutrons in an atom you get an isotope of the same element. Change in the number of protons changes the property of the entire atom and gives a different element.
Can you change the number of neutrons in a atom. 27 August 2021 by lets tokmak If you change the number of neutrons in an atom you will also. What particle in an atom never changes.
O number d isotope. The only way to change the number of protons in an atom is with a nuclear reaction. An example could be a carbon 14 atom decaying into carbon 12 atom.
If you change the number of protons in an atom somehow then there will be less positive charge. Since it has the same number of protons and electrons the total charge is 660. 3 What must change in an atom for it to become a new element.
If an atom has the same number of protons and neutrons it will have a neutral charge ie. The number of neutrons or electrons in an atom can change without changing the identity of the element. If you change the number of neutrons somehow nothing will happen because it carrys no charge at all.
B The particles that form an atom are equidistant from each other. Yw 3. Click to see full answer.
Meanwhile the chemical properties of the atom will not change. This means there are 6 neutrons approximately. What happens to an atom if we lose or gain protons neutrons or electrons.
This is due to the same number of valence electrons and therefore bonding and hybridisation will be the same. Therefore an elements atomic number will never change. The Atomic Number will change.
The amount of energy required to remove one is far greater than the energy typically found in chemical reactions. If the atomic mass reaches its upper limit then the decaying of the isotope will occur very quickly. No charge at all An example would be carbon-12.
What results from an unequal sharing of electrons between atoms. Yes You could add a thousand neutrons into the mix and the charge will not change. A cation B.
However if you add a thousand neutrons you will be creating one. The atomic number is the number of protons. Adding or losing neutrons will change the atomic mass without forming a different element.
Carbon would still be carbon but you would have changed its atomic mass. Which of the following is the best description of an atoms physical structure. The addition of a neutron can make an atom radioactive.
If you change the number of electrons somehow then there will be less negative charge. When you change the number of protons in an atom you will change the atom from one element to a different element. Why doesnt the number of protons change for a single element while the neutrons and electrons can.
Click to see full answer. 5 points QUESTION 7 1. Dec 14 2011 3 sjb-2812 445 5 Perhaps philosophically if you change the number of protons of an atom it no longer retains the identity of that atom.
4 When you change number of electrons on an you produce a an. The atomic number is the number of protons that is contained in the nucleus if you add a proton you change the element. If you change the number of neutrons in an atom you create _____.
An isotope D. A polar covalent bond C. The number of carbon isotopes doesnt change the atomic mass very much.
If you change the number of neutrons you wouldnt change the element of the atom. If there are many atoms of an element that are isotopes the average atomic mass for that element will change. 5 Which equation below would you use to determine the number of neutrons in a given nucleus.
If you change the number of neutrons in an atom you will also change its charge element. Meanwhile change in the number of electrons changes the atoms to ions which are chemically active. Both still have 6 protons but are isotopes since they have different numbers of neutrons.
Lets Answer The World. 5 points QUESTION 8 1. Hope this can help.
For example Carbons atomic numbernumber of protons is 6 and the mass number is 12011. Neutron numbers are able to change the mass of atoms because they weigh about as much as a proton and electron together. All it will affect is your average atomic mass which is the sum of protons and neutrons.
6 When you change the number of electrons the mass number changes. If you change the number of neutrons in an atom you get an isotope of the same element. Do note that you cannot endlessly add neutrons to an atom.
A a cation B an anion C an isotope D a different element. A nonpolar covalent bond B. The change of number of neutrons does not affect the charge of the atom.
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

Mass Number Easy Science Mass Number Number Definition Biology Notes
Comments
Post a Comment