Ionic Compound Formula for Barium Phosphate
Names of Some Polyatomic Ionic Compounds. This problem has been solved.
Experts are tested by Chegg as specialists in their subject area.
. Write the element symbols for barium and phosphide phosphide is phosphorus on the P-table. See the answer See the answer done loading. KC 2 H 3 O 2 potassium acetate.
Formula 21 sodium phosphide Na 3P 22 magnesium nitrate MgNO 32 23 lead II sulfite PbSO 3 24 calcium. 251 rows Calcium Phosphate. Monoisotopic mass 603622559 Da.
What is the ionic formula for barium and phosphate. For the following compounds give the formulas and the molar masses. NH 4 Cl ammonium chloride.
What is the ionic formula for barium and phosphate. It is an inorganic salt consists of. Molecular Formula Ba 3 O 8 P 2.
The strong attraction between positive and. We review their content and use your feedback to keep the quality high. Ionic compounds form when positive and negative ions share electrons and form an ionic bond.
Examples are shown in Table 35. Following are some of the chemical properties of barium phosphate. 8 rows Molecular Formula.
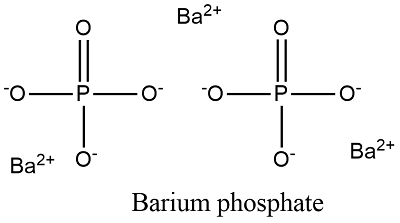
So barium phosphate would be Ba3 PO42. Diphosphate the barium phosphate formula is written as Ba 3 PO 4 2. Phosphate is a polyatomic ion with a charge of 3- PO4 3-.
State Some of the Physical Features of the Salt. The chemical formula of barium phosphate is Ba 3 PO 4 2. Ans- The IUPAC name given to the barium phosphate is barium2.
Average mass 601924 Da. Barium phosphate formula also known as Tribarium diphosphate formula or Barium phosphate tribasic formula is explained in this article. Refer to the explanation.
Barium oxide Barium oxide has the formula BaO. 86 7 ratings Transcribed image text. To form a compound that is electrically neutral one would need to use 3 barium ions total charge of 6 and 2 phosphate.
Barium phosphide is a binary ionic compound a metal a non-metal. Its an ionic compound so its formula represents the lowest whole number ratio of elements in the. So barium phosphate would be Ba3PO42.
The barium cation is Ba2. The phosphate anion is PO4 3-. The phosphate anion is PO4 3-.
7 rows Barium phosphite. ChemSpider ID 145305 - Charge. It has a molecular weight of 29527 gmol.
The barium cation is Ba2. Updated on January 02 2021. What is the correct formula for an ionic compound that contains barium ions and phosphate.
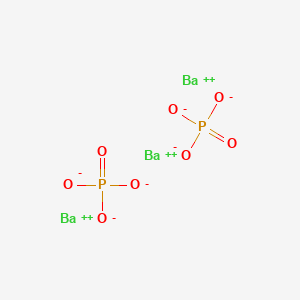
Barium Phosphate Ba3 Po4 2 Pubchem

How To Write The Formula For Barium Phosphate Youtube


Comments
Post a Comment